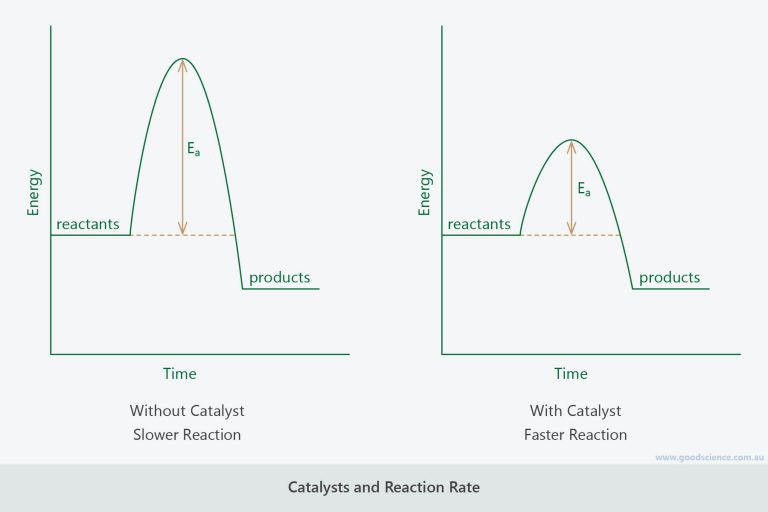
The Effect Of Catalysts On Rate Of Reaction . this page explains how adding a catalyst affects the rate of a reaction. Chart showing how the energy of. this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts. the effect of a catalyst on the activation energy is shown on a chart called a. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. chemical reaction rates increase or decrease according to factors including temperature, pressure and light. catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. It assumes that you are already familiar with basic ideas about the.
from www.goodscience.com.au
revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. this page explains how adding a catalyst affects the rate of a reaction. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. chemical reaction rates increase or decrease according to factors including temperature, pressure and light. the effect of a catalyst on the activation energy is shown on a chart called a. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. Chart showing how the energy of. It assumes familiarity with basic concepts. It assumes that you are already familiar with basic ideas about the.
Factors that Affect Rate of Reaction Good Science
The Effect Of Catalysts On Rate Of Reaction this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts. this page describes and explains the way that adding a catalyst affects the rate of a reaction. the effect of a catalyst on the activation energy is shown on a chart called a. It assumes that you are already familiar with basic ideas about the. Chart showing how the energy of. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. chemical reaction rates increase or decrease according to factors including temperature, pressure and light. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. this page explains how adding a catalyst affects the rate of a reaction.
From courses.lumenlearning.com
Catalysis General Chemistry The Effect Of Catalysts On Rate Of Reaction describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. the effect of a catalyst on the activation energy is shown on a chart called a. chemical reaction rates increase or decrease according to factors including temperature, pressure and light. revise how to measure rates of reaction and how to impact the rate. The Effect Of Catalysts On Rate Of Reaction.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog The Effect Of Catalysts On Rate Of Reaction catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. Chart showing how the energy of. It assumes familiarity with basic concepts. the effect of a catalyst on the activation energy is shown on a chart called a. this page explains how adding a catalyst affects the rate of a reaction. revise. The Effect Of Catalysts On Rate Of Reaction.
From www.slideshare.net
1.4 rate of reaction(1.2d)...biology The Effect Of Catalysts On Rate Of Reaction this page explains how adding a catalyst affects the rate of a reaction. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. chemical reaction rates increase or decrease according to factors including temperature, pressure and light. the effect of a catalyst on the activation energy is shown on a chart called a.. The Effect Of Catalysts On Rate Of Reaction.
From www.researchgate.net
Effect of catalyst dose on the apparent rate constant of reaction. At... Download Scientific The Effect Of Catalysts On Rate Of Reaction the effect of a catalyst on the activation energy is shown on a chart called a. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. It assumes that you are already familiar with basic ideas about the. Chart showing how the energy of. this page explains how adding a catalyst affects the rate. The Effect Of Catalysts On Rate Of Reaction.
From www.youtube.com
Effect of catalyst on rate of reaction YouTube The Effect Of Catalysts On Rate Of Reaction the effect of a catalyst on the activation energy is shown on a chart called a. It assumes that you are already familiar with basic ideas about the. chemical reaction rates increase or decrease according to factors including temperature, pressure and light. this page describes and explains the way that adding a catalyst affects the rate of. The Effect Of Catalysts On Rate Of Reaction.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry The Effect Of Catalysts On Rate Of Reaction describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. the effect of a catalyst on the activation energy is shown on a chart called a. chemical reaction rates increase or decrease according. The Effect Of Catalysts On Rate Of Reaction.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 The Effect Of Catalysts On Rate Of Reaction describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. this page describes and explains the way that adding a catalyst affects the rate of a reaction. chemical reaction rates increase or decrease according to factors including temperature, pressure and light. the effect of a catalyst on the activation energy is shown on. The Effect Of Catalysts On Rate Of Reaction.
From www.youtube.com
Catalysis Catalyst Effect of Catalysts on rate of reaction B.Sc. 1st year chemistryAK The Effect Of Catalysts On Rate Of Reaction this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. the effect of a catalyst on the activation energy is shown on a chart. The Effect Of Catalysts On Rate Of Reaction.
From www.researchgate.net
Effect of catalyst concentration on the rate of reaction , MSA; , pTSA Download Scientific The Effect Of Catalysts On Rate Of Reaction revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. Chart showing how the energy of. the effect of a catalyst on the activation energy is shown on a chart called a. chemical. The Effect Of Catalysts On Rate Of Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii The Effect Of Catalysts On Rate Of Reaction revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. this page describes and explains the way that adding a catalyst affects the rate of a reaction. catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. chemical reaction rates increase or. The Effect Of Catalysts On Rate Of Reaction.
From www.youtube.com
Lecture 19 Effect of catalyst on rate of reaction YouTube The Effect Of Catalysts On Rate Of Reaction this page describes and explains the way that adding a catalyst affects the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. revise how to measure rates of reaction and how to impact the rate by changing. The Effect Of Catalysts On Rate Of Reaction.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram The Effect Of Catalysts On Rate Of Reaction It assumes familiarity with basic concepts. It assumes that you are already familiar with basic ideas about the. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. the effect of a catalyst on the activation energy is shown on a chart called a. catalysts increase the frequency of collisions between reactants, alter molecular. The Effect Of Catalysts On Rate Of Reaction.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog The Effect Of Catalysts On Rate Of Reaction chemical reaction rates increase or decrease according to factors including temperature, pressure and light. Chart showing how the energy of. catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. the effect of a catalyst on the activation energy is shown on a chart called a. this page explains how adding a. The Effect Of Catalysts On Rate Of Reaction.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog The Effect Of Catalysts On Rate Of Reaction this page explains how adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. It assumes familiarity with basic concepts. Chart showing how the energy of. this page describes and explains the way that adding a catalyst affects the rate of a reaction. describe how temperatures,. The Effect Of Catalysts On Rate Of Reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube The Effect Of Catalysts On Rate Of Reaction catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. Chart showing how the energy of. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. the effect of a catalyst on the activation energy is shown on a chart called a. this page explains how adding a catalyst. The Effect Of Catalysts On Rate Of Reaction.
From www.youtube.com
Effect of catalyst on the rate of reaction YouTube The Effect Of Catalysts On Rate Of Reaction catalysts increase the frequency of collisions between reactants, alter molecular orientation, reduce intermolecular bonding. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. the effect of a catalyst on the activation energy is shown on a chart called a. this page describes and explains the way that adding a catalyst affects the. The Effect Of Catalysts On Rate Of Reaction.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID883534 The Effect Of Catalysts On Rate Of Reaction It assumes that you are already familiar with basic ideas about the. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. chemical reaction rates increase or decrease according to factors including temperature, pressure and light. Chart showing how the energy of. this page describes and explains. The Effect Of Catalysts On Rate Of Reaction.
From www.youtube.com
Effect of Catalyst on Rate of Reaction Chemical Chemistry Class 12 YouTube The Effect Of Catalysts On Rate Of Reaction the effect of a catalyst on the activation energy is shown on a chart called a. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. this page explains how adding a catalyst. The Effect Of Catalysts On Rate Of Reaction.